Neurocrine Biosciences Reports Positive Phase II Data for Crinecerfont in Adults with Congenital Adrenal Hyperplasia at ENDO Online 2020
Neurocrine Biosciences Reports Positive Phase II Data for Crinecerfont in Adults with Congenital Adrenal Hyperplasia at ENDO Online 2020
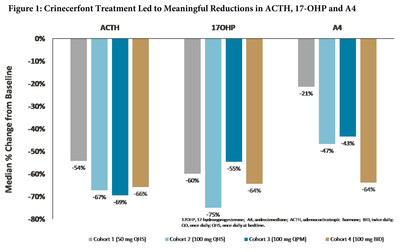
- Crinecerfont Demonstrates Meaningful Reductions in Key Disease Biomarkers After 14 Days of Treatment
- Initiation of Global Registrational Study of Crinecerfont in Adults Planned for Second Half 2020
Table 1: Number and Percentage of Participants in Each Cohort Demonstrating a ≥ 50% Reduction from Baseline in ACTH, 17-OHP and Androstenedione (A4)
|
≥ 50% reduction from baseline, n/N (%) |
Cohort 1: 50 mg QHS |
Cohort 2: 100 mg QHS |
Cohort 3: 100 mg QPM |
Cohort 4: 100 mg BID |
|
ACTH |
4/8 (50.0) |
5/6 (83.3) |
5/7 (71.4) |
6/8 (75.0) |
|
17-OHP |
4/8 (50.0) |
4/6 (66.7) |
4/7 (57.1) |
6/8 (75.0) |
|
Androstenedione (A4) |
2/8 (25.0) |
3/6 (50.0) |
3/7 (42.9) |
6/8 (75.0) |
"There continues to be a need for effective treatment options that are well tolerated in patients with classic CAH. Patients with this genetic disorder require glucocorticoid replacement therapy, but often at high doses to manage their excessive adrenal androgen production. At the same time, side effects from chronic treatment with supraphysiologic amounts of glucocorticoids can cause serious long-term health consequences including bone loss and metabolic dysfunction," said
"We are pleased that crinecerfont was effective in producing a meaningful, dose-related reduction of adrenal androgens and other key biomarkers of disease in patients with classic CAH and was well tolerated in this study," said Eiry W. Roberts, M.D., Chief Medical Officer at
Crinecerfont Phase II Study Design
The Phase II open-label, multiple-dose, dose-finding study assessed the safety, tolerability, pharmacokinetics and pharmacodynamics of crinecerfont in 18 adults with classic 21-hydroxylase deficiency CAH. The study's sequential-cohort design evaluated four crinecerfont oral dosing regimens: 50 mg at bedtime (Cohort 1; n=8); 100 mg at bedtime (Cohort 2; n=7); 100 mg once-daily with an evening meal (Cohort 3; n=8); and 100 mg twice-daily with meals (Cohort 4; n=8). Participants in Cohorts 1 and 2 could enroll in Cohorts 3 and/or 4. Each regimen was administered for 14 consecutive days. ACTH, 17-OHP and A4, key disease hormone markers in CAH patients, were measured over a 24-hour period at baseline and after 14 consecutive days of dosing.
About Classic Congenital Adrenal Hyperplasia (CAH)
Classic CAH is a genetic disorder, in which an enzyme deficiency alters the production of adrenal steroids. Because of this deficiency, the adrenal glands fail to produce enough cortisol and, sometimes, aldosterone, resulting in a potentially life-threatening condition. The lack of cortisol stimulates the release of high levels of adrenocorticotropic hormone (ACTH) from the pituitary gland, leading to excessive adrenal androgen levels. These levels can lead to virilization, menstrual irregularities, hirsutism, acne in females and accelerated growth and precocious puberty in childhood (resulting in short stature and fertility problems in both males and females).
Corticosteroids, the current standard of care, are used both to correct the endogenous cortisol deficiency and to reduce the high ACTH levels and androgen excess. However, the dose and duration of glucocorticoids required to suppress ACTH are often well above the normal physiological level of cortisol, which can result in serious complications typical of iatrogenic Cushing's syndrome, including metabolic issues, bone loss, growth impairment, and infection risk. Classic CAH is a disease that affects approximately 20,000 to 30,000 people in
About Crinecerfont
Crinecerfont is a novel, potent, selective, oral, non-steroidal corticotropin-releasing factor type 1 (CRF1) receptor antagonist under evaluation for the treatment of classic CAH. The blockade of CRF receptors in the pituitary has been shown to decrease the release of adrenocorticotropic hormone (ACTH), which in turn decreases the production of adrenal androgens, and potentially the symptoms associated with CAH. Lowering ACTH and adrenal androgen levels could reduce the amount of glucocorticoid treatment necessary for disease control and thus could avoid the complications associated with long-term supraphysiologic glucocorticoid therapy.
About
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include statements regarding the potential benefits of crinecerfont to patients and future clinical development plans. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements include the risk that crinecerfont will not be found to be safe and/or effective or may not prove to be beneficial to patients; that development activities for crinecerfont may not be completed on time or at all; risks that clinical development activities may be delayed for regulatory or other reasons, may not be successful or replicate previous and/or interim clinical trial results, or may not be predictive of real-world results or of results in subsequent clinical trials; risks that regulatory submissions for crinecerfont may not occur or be submitted in a timely manner; risks that crinecerfont may not obtain regulatory approvals; or that the
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/neurocrine-biosciences-reports-positive-phase-ii-data-for-crinecerfont-in-adults-with-congenital-adrenal-hyperplasia-at-endo-online-2020-301072072.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/neurocrine-biosciences-reports-positive-phase-ii-data-for-crinecerfont-in-adults-with-congenital-adrenal-hyperplasia-at-endo-online-2020-301072072.html
SOURCE
Navjot Rai (Media), 858-617-7623, media@neurocrine.com; Todd Tushla (Investors), 858-617-7143, ir@neurocrine.com