Neurocrine Biosciences® Presents INGREZZA® (valbenazine) Capsules Interim Data Demonstrating Sustained Improvements in Chorea Associated With Huntington's Disease Through Week 50 at Huntington Study Group 2023
"These interim data provide insight on the clinically meaningful and sustained improvements participants are experiencing with INGREZZA for the treatment of chorea," said Eiry W. Roberts, M.D., Chief Medical Officer at
KINECT-HD2 includes adults with genetically confirmed motor-manifest HD (n=127), most of whom (n=98) completed KINECT-HD, a Phase 3, randomized, double-blind, placebo-controlled study. Both studies were conducted in collaboration with the
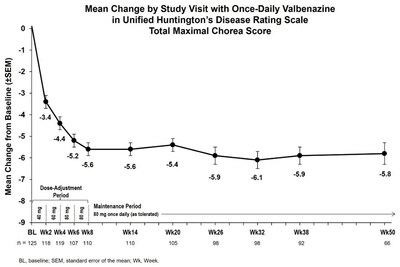
The current interim results from KINECT-HD2 (Sustained Improvements With Once-Daily Valbenazine in Chorea Associated With Huntington's Disease: Interim Results From a Long-Term Open-Label Study, Poster # 64) suggest that INGREZZA improved chorea at the first evaluation (Week 2) when participants were taking the lowest dose of 40 mg, with efficacy sustained through Week 50 at ≤ 80 mg (Figure 1).
More than half of participants (60.9 percent) and investigators (58.9 percent) rated symptoms as "much improved" or "very much improved" at Week 6, and about three-quarters of participants (74.2 percent) and investigators (76.9 percent) rated symptoms as "much improved" or "very much improved" by Week 50. The most common treatment-emergent adverse events at the time of the analysis were consistent with those observed in KINECT-HD, including falls (30.4 percent), fatigue (24.0 percent) and somnolence (24.0 percent).
Additional HD chorea presentations at the 30th Annual Meeting of the HSG include:
- The
Huntington's Disease Health Index (HD-HI): Measuring Changes in Disease Burden in Response to Valbenazine During the KINECT®-HD Trial (Poster #66) - A Minimal Clinically Important Difference for UHDRS® Total Maximal Chorea Score as a Measure of Chorea Severity in
Huntington's Disease (Poster #63) - Indirect Treatment Comparison of Valbenazine With Deutetrabenazine for Improvement in Total Maximal Chorea Score in
Huntington's Disease (Poster #62)
The full abstracts can be accessed on the
About Chorea Associated with Huntington's Disease (HD)
About KINECT®-HD
KINECT®-HD was a Phase 3, randomized, double-blind, placebo-controlled study designed to evaluate the efficacy of valbenazine as a once-daily treatment to reduce chorea associated with Huntington's disease (HD) and evaluate the safety and tolerability of valbenazine in patients with HD. The study enrolled 128 adults 18 to 75 years of age who were diagnosed with motor-manifest HD and who had sufficient chorea symptoms to meet study protocol criteria.
KINECT-HD used the Unified Huntington's Disease Rating Scale (UHDRS®) Total Maximal Chorea (TMC) score as the primary efficacy endpoint. The secondary endpoints included Clinical Global Impression of Change (CGI-C) response status and Patient Global Impression of Change (PGI-C) response status for valbenazine treatment. Treatment with valbenazine resulted in a placebo-adjusted mean reduction in the TMC score of 3.2 units (P < 0.0001), indicating a substantial improvement in chorea. Secondary endpoints of CGI-C response status and PGI-C response status were also statistically significant and supported the improvements in TMC score that were seen over the 12-week study period.
Treatment-emergent adverse events in this study were generally consistent with the known safety profile of valbenazine. The most common adverse reactions in patients with HD included somnolence and sedation, urticaria, rash and insomnia.
View the complete study results from the Phase 3 KINECT-HD study published in The Lancet Neurology online edition. For more information on the KINECT-HD study, please visit HuntingtonStudyGroup.org.
About KINECT®-HD2
KINECT®-HD2 is an ongoing open-label study to evaluate the long-term safety and tolerability, as well as the maintenance of effects, of INGREZZA in patients with chorea associated with Huntington's disease (HD). The 156-week study has enrolled more than 150 adults 18 to 75 years of age who have been diagnosed with motor-manifest HD and who have sufficient chorea symptoms to meet study protocol criteria. Concomitant antipsychotic use is allowed in the study. For more information on the KINECT-HD2 study, please visit HuntingtonStudyGroup.org or ClinicalTrials.gov.
About Huntington Study Group / HSG Clinical Research, Inc.
About INGREZZA® (valbenazine) Capsules
INGREZZA is the only one-capsule, once-daily selective vesicular monoamine transporter 2 (VMAT2) inhibitor approved by the U.S. Food and Drug Administration for the treatment of adults with tardive dyskinesia and the treatment of chorea associated with Huntington's disease (HD).
INGREZZA, developed by
Important Information
Approved Uses
INGREZZA® (valbenazine) capsules is a prescription medicine used to treat adults with:
- movements in the face, tongue, or other body parts that cannot be controlled (tardive dyskinesia).
- involuntary movements (chorea) of
Huntington's disease. INGREZZA does not cure the cause of involuntary movements, and it does not treat other symptoms ofHuntington's disease, such as problems with thinking or emotions.
It is not known if INGREZZA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
VMAT2 inhibitors, including INGREZZA, can cause serious side effects in people with
Do not take INGREZZA if you:
- are allergic to valbenazine, or any of the ingredients in INGREZZA.
INGREZZA may cause serious side effects, including:
- Sudden swelling from an allergic reaction (angioedema). Sudden swelling has happened after the first dose or after many doses of INGREZZA. Signs and symptoms of angioedema include: swelling of your face, lips, throat, and other areas of your skin, difficulty swallowing or breathing, and raised, red areas on your skin (hives). Swelling in the throat can be life-threatening and can lead to death. Go to the nearest emergency room right away if you develop these signs and symptoms. Your healthcare provider should stop your treatment with INGREZZA.
- Heart rhythm problems (QT prolongation). INGREZZA may cause a heart problem known as QT prolongation. Symptoms of QT prolongation may include: fast, slow, or irregular heartbeat, dizziness or fainting, or shortness of breath.
Tell your healthcare provider right away if you have a change in your heartbeat (a fast or irregular heartbeat), or if you faint.
- Neuroleptic Malignant Syndrome (NMS): NMS is a serious condition that can lead to death. Call a healthcare provider right away or go to the nearest emergency room if you develop these symptoms and they do not have another obvious cause: high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, or increased sweating.
- Abnormal movements (Parkinson-like). Symptoms include: shaking, body stiffness, trouble moving or walking, or keeping your balance.
Before taking INGREZZA, tell your healthcare provider about all of your medical conditions including if you: have liver or heart problems, are pregnant or plan to become pregnant, or are breastfeeding or plan to breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Sleepiness (sedation) is a common side effect with INGREZZA. While taking INGREZZA, do not drive a car or operate dangerous machinery until you know how INGREZZA affects you. Drinking alcohol and taking other drugs that may also cause sleepiness while you are taking INGREZZA may increase any sleepiness caused by INGREZZA.
The most common side effect of INGREZZA in people with tardive dyskinesia is sleepiness (somnolence).
The most common side effects of INGREZZA in people with
These are not all of the possible side effects of INGREZZA. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see INGREZZA full Prescribing Information, including Boxed Warning.
About Neurocrine Biosciences
Neurocrine Biosciences is a leading neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs, but few options. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine, and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, chorea associated with Huntington's disease, Parkinson's disease, endometriosis* and uterine fibroids*, as well as a robust pipeline including multiple compounds in mid- to late-phase clinical development across our core therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders, because you deserve brave science. For more information, visit Neurocrine.com, and follow the company on LinkedIn, X (formerly Twitter) and Facebook. (*in collaboration with AbbVie)
NEUROCRINE,
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements regarding the potential benefits to be derived from INGREZZA for the treatment of chorea associated with Huntington's disease (HD), and the value INGREZZA for the treatment of chorea associated with HD brings to patients. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements include: risks and uncertainties associated with Neurocrine Biosciences' business and finances in general, as well as risks and uncertainties associated with the commercialization of INGREZZA for the treatment of chorea associated with HD; whether INGREZZA for the treatment of chorea associated with HD receives adequate reimbursement from third-party payors; the degree and pace of market uptake of INGREZZA for the treatment of chorea associated with HD; risks and uncertainties relating to competitive products and technological changes that may limit demand for INGREZZA for the treatment of chorea associated with HD; risks associated with the Company's dependence on third parties for development and manufacturing activities related to INGREZZA for the treatment of chorea associated with HD, and the ability of the Company to manage these third parties; risks that additional regulatory submissions for INGREZZA for the treatment of chorea associated with HD or other product candidates may not occur or be submitted in a timely manner; risks that the FDA or other regulatory authorities may make adverse decisions regarding INGREZZA for the treatment of chorea associated with HD; risks that post-approval INGREZZA for the treatment of chorea associated with HD commitments or requirements may be delayed; risks that INGREZZA for the treatment of chorea associated with HD may be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; risks and uncertainties relating to competitive products and technological changes that may limit demand for INGREZZA for the treatment of chorea associated with HD; and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission, including without limitation the Company's quarterly report on Form 10-Q for the quarter ended June 30, 2023. Neurocrine Biosciences disclaims any obligation to update the statements contained in this press release after the date hereof.
©2023
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-presents-ingrezza-valbenazine-capsules-interim-data-demonstrating-sustained-improvements-in-chorea-associated-with-huntingtons-disease-through-week-50-at-huntington-study-group-2023-301969545.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-presents-ingrezza-valbenazine-capsules-interim-data-demonstrating-sustained-improvements-in-chorea-associated-with-huntingtons-disease-through-week-50-at-huntington-study-group-2023-301969545.html
SOURCE
Neurocrine Biosciences, Inc. Media: Aimee White, 1-858-354-7865, media@neurocrine.com; Investors: Todd Tushla, 1-858-617-7143, ir@neurocrine.com